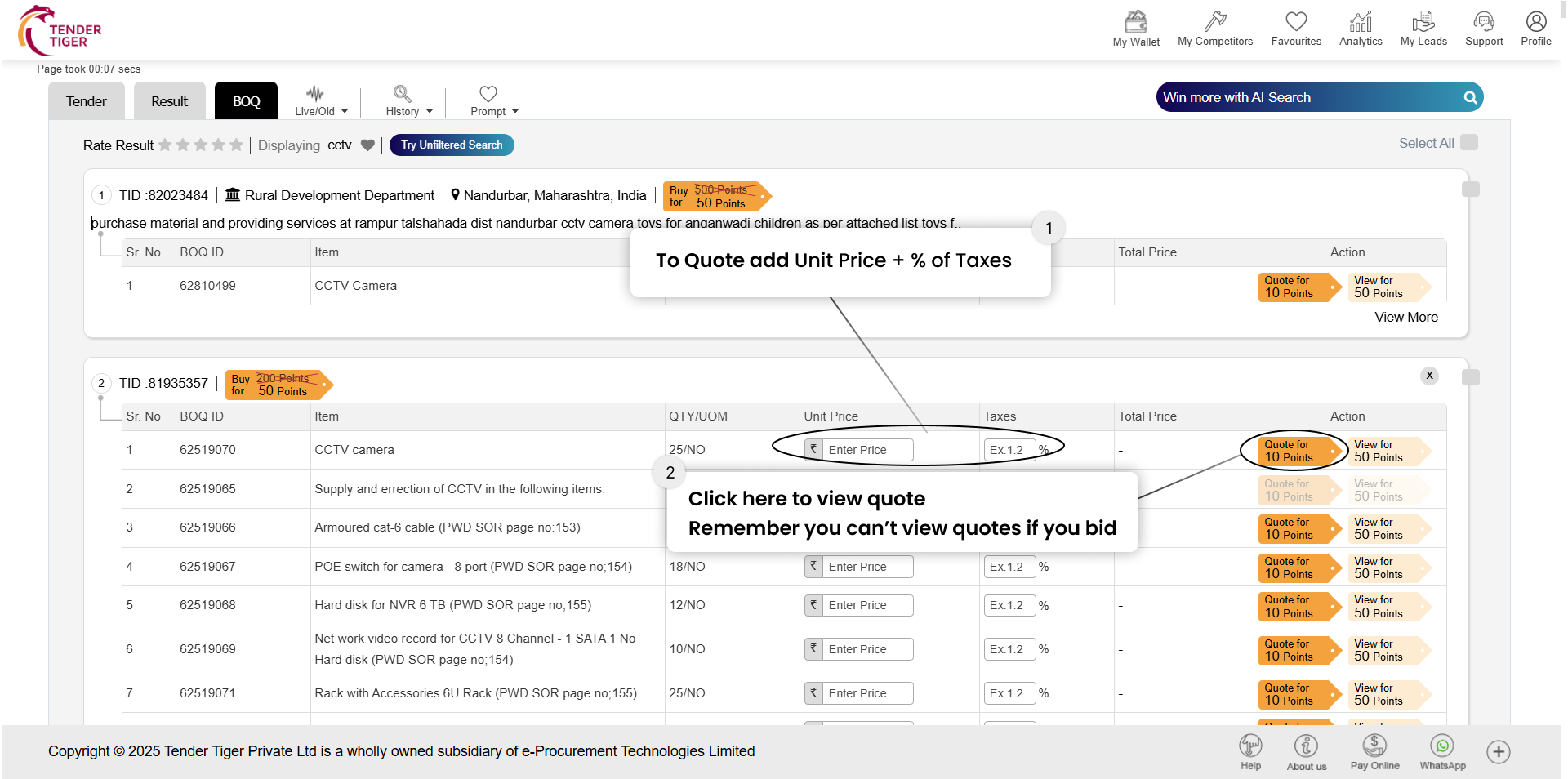
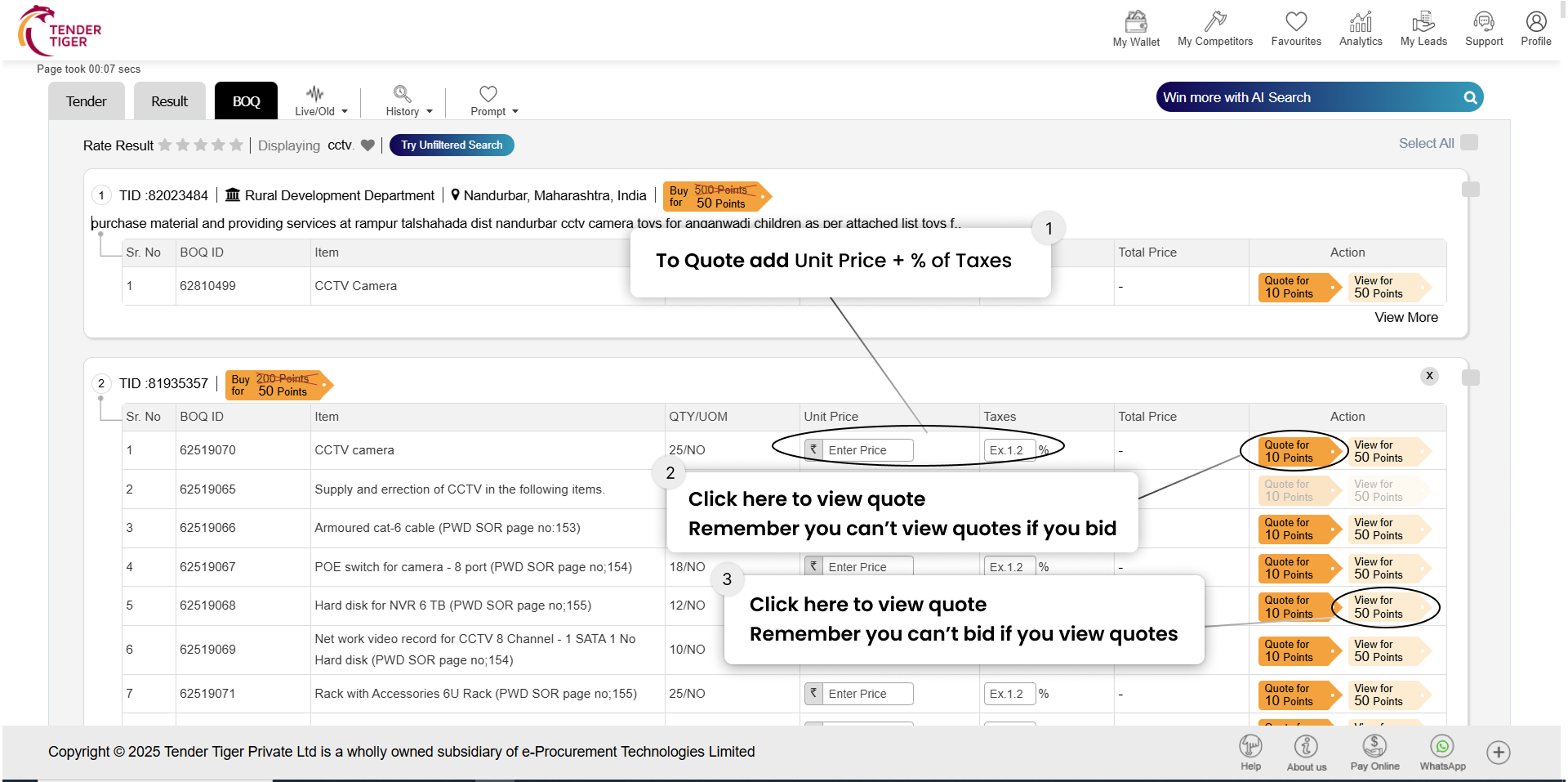
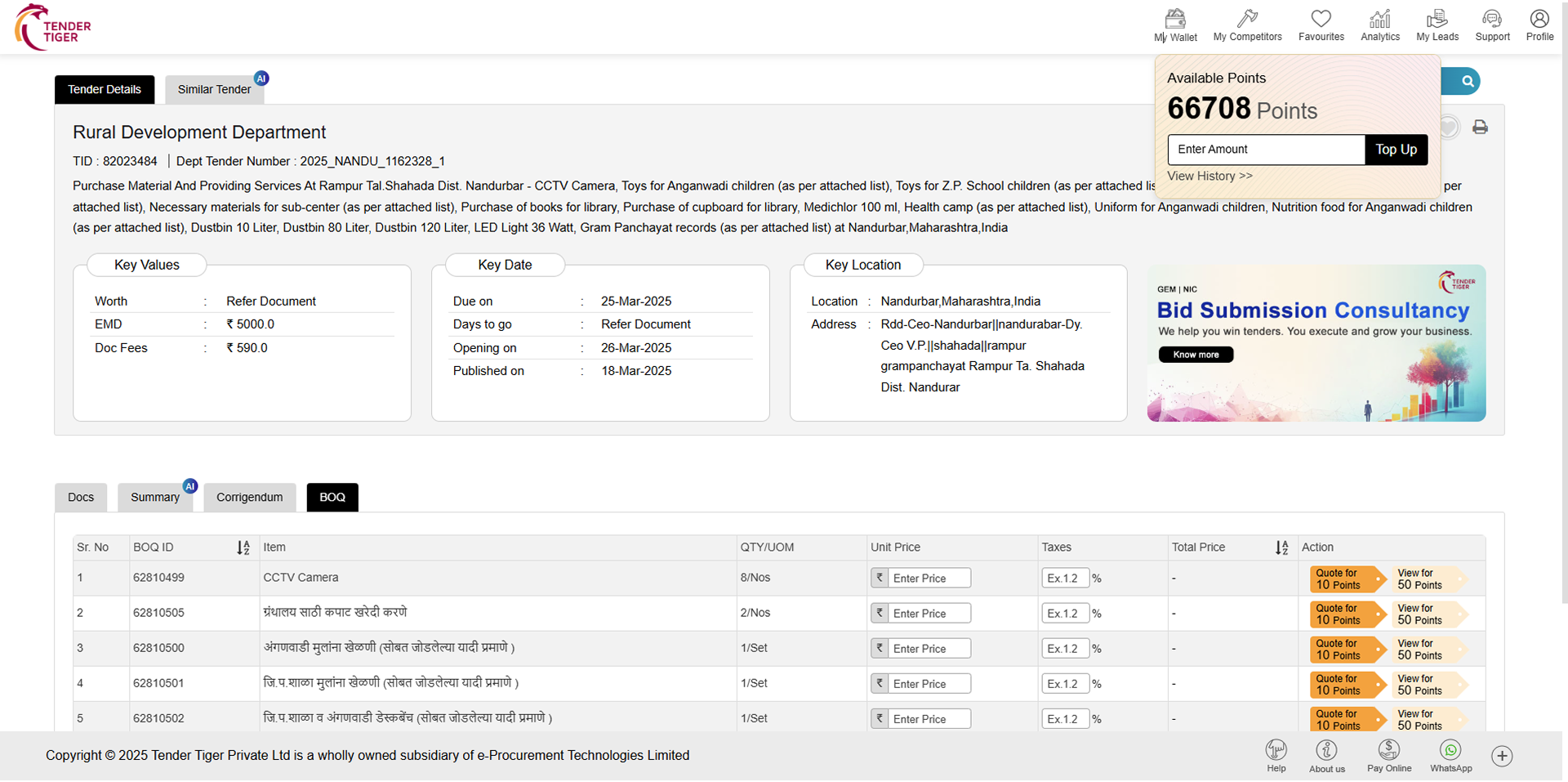
TRID :
8537299
Actual Tender Number :

TEC
TEC
TEC
Supply of drugs and dressings
"5-aminosalicylic acid (mesalazine/ mesalaine) retention enema:, each 60ml unit of rectal suspension contains mesalazine/mesalamine 4.0 grams , packing:60ml bottle", "mesalazine/ mesalaine suppository-, each suppository to contain: mesalazine/ mesalaine 500mg ", "mesalamine/ mesalazine tablet- , each tab to contain: mesalamine/ mesalazine 400mg.", "mercaptopurine tablet- , each tab to contain: mercaptopurine 50mg", abacavir + lamivudine tablet: each tab to contain: abacavir 60 mg + lamivudine 30 mg, abacavir + lamivudine tablet: each tab to contain: abacavir 600 mg + lamivudine 300 mg, abacavir tablet: each tablet to contain: abacavir 300 mg, abacavir tablet: each tablet to contain: abacavir 60 mg, "acetyl salicylic acid tab- , each enteric coated tablet to contain: acetyl salicylic acid 100 mg", "actinomycin d inj- , each vial to contain: actinomycin d 0.5mg, packing:1 vial ", "activated charcoal powder, packing:100 gm pack", "acyclovir inj- , each vial to contain: acyclovir 250mg, packing:1 vial", "acyclovir oral suspension-, each 5ml to contain: acyclovir 400 mg, , packing:100 ml bottle", "acyclovir tab- , each tablet to contain: acyclovir 800mg.", albendazole chewable tablet-each chewable tablet to contain albendazole 400mg , "allopurinol tab - , each tablet to contain: allopurinol 300 mg", all-trans retinoic acid cap-each capsule to contain all trans retinoic acid 10mg, "alprostadil inj- each ml to contain alprostadil 500mcg, packing:1ml vial/amp", "amitriptyline tab- , each tablet to contain: amitriptyline 50mg", "amitriptyline tab- , each tablet to contain: amitriptyline 75mg", amlodipine tab- each tablet to contain: amlodipine 10mg, amlodipine tab- each tablet to contain: amlodipine 2.5 mg, "amoxycillin oral suspension - each 5 ml to contain :amoxycillin 250 mg, , packing:100ml bottle", "amoxicillin inj- , each vial to contain: amoxicillin 1000 mg, packing:1 vial", "amoxicillin inj- , each vial to contain: amoxicillin 250 mg, packing:1 vial", "amoxicillin inj- , each vial to contain: amoxicillin 500 mg, packing:1 vial", "lipid amphotericin b inj- , each vial to contain: lipid amphotericin b 50 mg, packing:1 vial", "ampicillin inj- , each vial to contain: ampicillin 1000 mg(1gm), packing:1 vial", "arsenic trioxide inj-each ml to contain arsenic trioxide 1mg, packing:10ml vial", "artemether & lumefantrine tablet- , each tablet to contain: artemether 40mg and lumefantrine 240mg", "anti-malarial kit- , each combikit to contain: (a) 1 tablet of artesunate 150mg (b)2 tablets of sulphadoxine 500mg & pyrimethamine 25mg ", "anti-malarial kit- , each combikit to contain: (a) 1 tablet of artesunate 25mg (b) 1 tablet of sulphadoxine 250mg & pyrimethamine 12.5mg ", "anti-malarial kit- , each combikit to contain: (a) 1 tablet of artesunate 50mg (b) 1 tablet of sulphadoxine 500mg & pyrimethamine 25mg ", "anti-malarial kit- , each combikit to contain: (a) 1 tablet of artesunate 100mg (b) 1 tablet of sulphadoxine 750mg & pyrimethamine 37.5mg ", "artesunate inj- each vial to contain:artesunate 120 mg, packing:1 vial", ascorbic acid (vitamin c) tablet - each tablet to contain ascorbic acid 100mg, atazanavir + ritonavir tab-each tablet to contain atazanavir 300 mg+ritonavir 100 mg , atorvastatin 40 mg tab - each tablet to contain: atorvastatin 40 mg , atorvastatin 80 mg tab - each tablet to contain: atorvastatin 80 mg , "atropine eye drop: each ml to contain atropine 1% , packing:5ml vial", "azathioprine tab- , each tablet to contain: azathioprine 25 mg", azithromycin tab. - each tablet to contain: azithromycin 1000mg, "baclofen tab-, each tablet to contain: baclofen 20 mg ", "baclofen tab-, each tablet to contain: baclofen 5 mg ", "barium sulphate oral liquid-barium sulphate 95% w/v, , packing:500ml bottle, ", "bedaquilinetab-, each tablet to contain: bedaquiline 100 mg", "bendamustine inj-, each vial to contain: bendamustine hydrochloride 25mg, packing:1 vial", "betamethasone valerate cream- each gm to contain betamethasone valerate 0.05 % , packing:15gm tube", "betamethasone valerate cream- each gm to contain betamethasone valerate 0.1% , packing:15gm tube", "bisacodyl suppository- , each suppository to contain: bisacodyl 5mg", "bisacodyl tab-- , each tablet to contain: bisacodyl 5 mg", "bortezomib inj- , each vial to contain: bortezomib 2 mg, packing:1 vial ", "metered dose inhaler - , each dose to contain: formoterol 6 mcg, budesonide 100 mcg , , packing:equal to or more than 120 mdi", "metered dose inhaler - , each dose to contain: formoterol 6 mcg, budesonide 400 mcg , , packing:equal to or more than 120 mdi", "budesonide inhaler- , each metered dose to contain : budesonide 200 mcg, packing:equal to or more than 120 mdi", "budesonide nasal spray- , each actuation to deliver: budesonide 50 mcg., packing:100 to 150 doses", "budesonide respirator solution for use in nebulizer: each ml to contain budesonide 0.5 mg, packing:2ml respule", "budesonide respirator solution for use in nebulizer: each ml to contain budesonide 1 mg, packing:2ml respule", "bupivacaine injection 0.5 % with 7.5 % glucose, packing:4 ml amp", buprenorphine and naloxone tablet-each sub-lingual tablet contain buprenorphine 0.4 mg and naloxone 0.1 mg., buprenorphine and naloxone tablet-each sub-lingual tablet contain buprenorphine 2 mg and naloxone 0.5 mg, buprenorphine tablet-each sub-lingual tablet contain buprenorphine 0.4 mg, "caffeine inj- , each ml to contain: caffeine citrate 20mg, packing: 2 ml vial/amp", "caffeine oral solution- each ml to contain: caffeine citrate 20mg , packing: 3 ml bottle", calcium carbonate tablet- each tablet to contain calcium carbonate 1250 mg (equivalent to elemental calcium 500 mg) , calcium carbonate tablet- each tablet to contain calcium carbonate 625 mg (equivalent to elemental calcium 250 mg) , "calcium folinate inj- , each ml to contain: calcium folinate 3mg, packing: 1 ml vial/amp", "calcium folinate tab- , each tab to contain: calcium folinate 15mg", "carbamazepine oral liquid- , each 5ml to contain: carbamazepine 100mg, , packing:100 ml bottle", "carbamazepinetab- , each tab to contain: carbamazepine 100mg", "carbamazepinetab- , each tablet to contain: carbamazepine 400mg", "carbimazole tab- , each tab to contain: carbimazole 10 mg.", "carbimazole tab- , each tab to contain: carbimazole 20 mg.", "carboxy methyl cellulose eye drop- , each ml to contain: carboxy methyl cellulose 10mg, packing: 10 ml vial", "cefadroxil oral liquid-each 5ml to contain cefadroxil 125mg , , packing:30ml bottle", cefadroxil tab - each tablet to contain: cefadroxil 100mg, "cefazolin inj-each vial to contain cefazolin 1000 mg, packing: 1 vial", "cefazolin inj-each vial to contain cefazolin 500 mg, packing: 1 vial", "cefixime oral liquid - , each 5ml to contain: cefixime 100mg, , packing:30ml bottle", cefixime tab. - each tablet to contain: cefixime 400mg, "ceftriaxone inj. - each vial to contain: ceftriaxone 2000mg, packing: 1 vial", chlorambucil tab. - each tablet to contain : chlorambucil 2mg, chlorambucil tab. - each tablet to contain : chlorambucil 5mg, "chlorhexidine concentrate soln- , each bott to contain: chlorhexidine 5% , , packing: 1 litre bottle", chloroquine tab. - each tablet to contain: chloroquine 150mg, "cholecalciferol - , each tab/cap to contain :cholecalciferol 1000 iu", "ciprofloxacin oral suspension- , after reconstitution each 5ml to contain: ciprofloxacin 250mg, , packing:60ml bottle", "ciprofloxacin eye oint: contain ciprofloxacin 0.3% , packing:5gm tube", "clarithromycin oral liquid- , each 5ml to contain: clarithromycin 125mg , , packing:30ml bottle", "clarithromycin tab- , each tab to contain: clarithromycin 250mg", "clarithromycin tab- , each tab to contain: clarithromycin 750mg", clindamycin cap- each capsule to contain: clindamycin 150mg, "clofazimine cap - , each capsule to contain: clofazimine 50mg", "clomiphene citrate tab- , each tab to contain: clomiphene citrate 100 mg", "clomipramine cap- , each capsu at
Classified in :