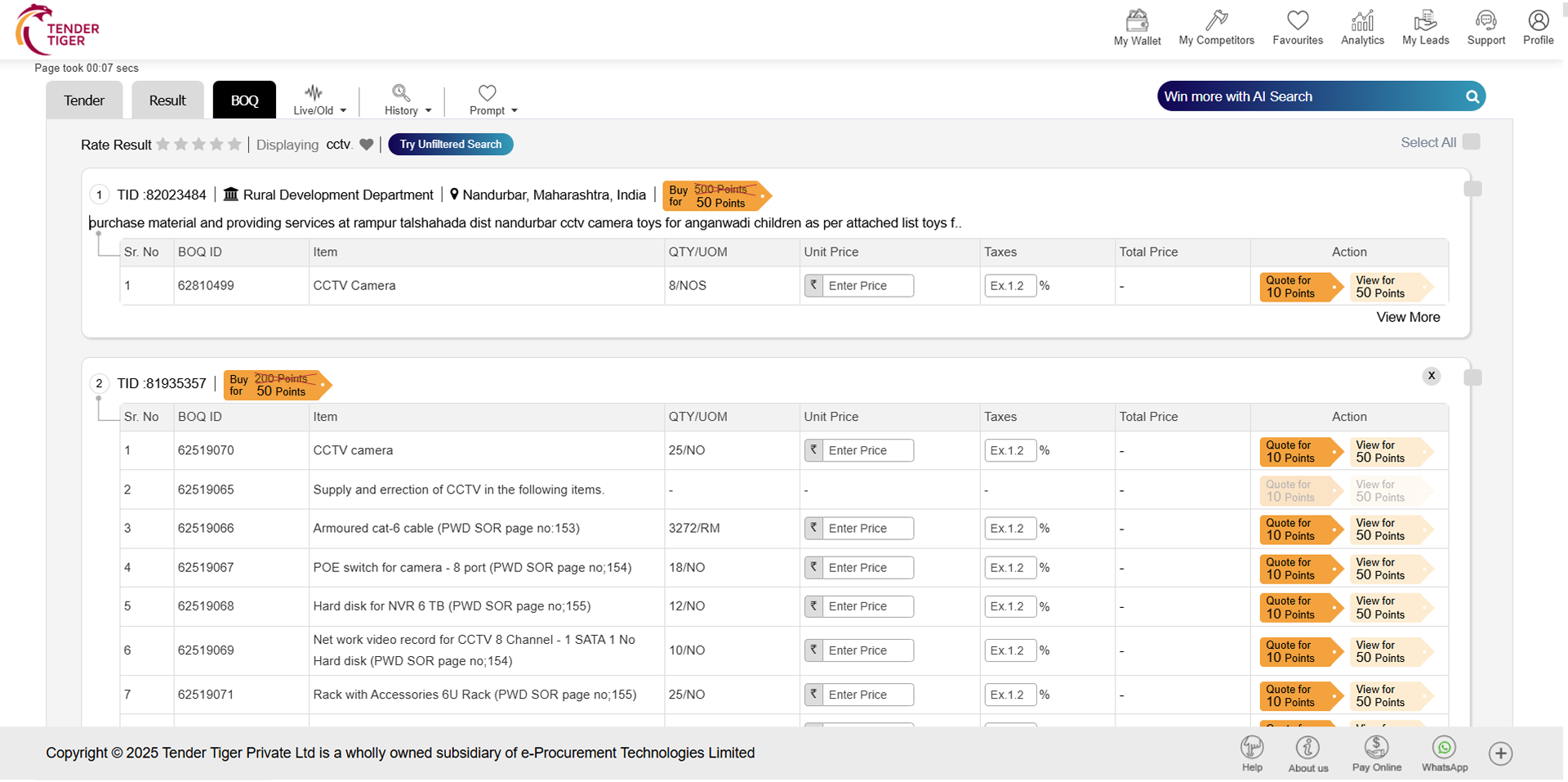
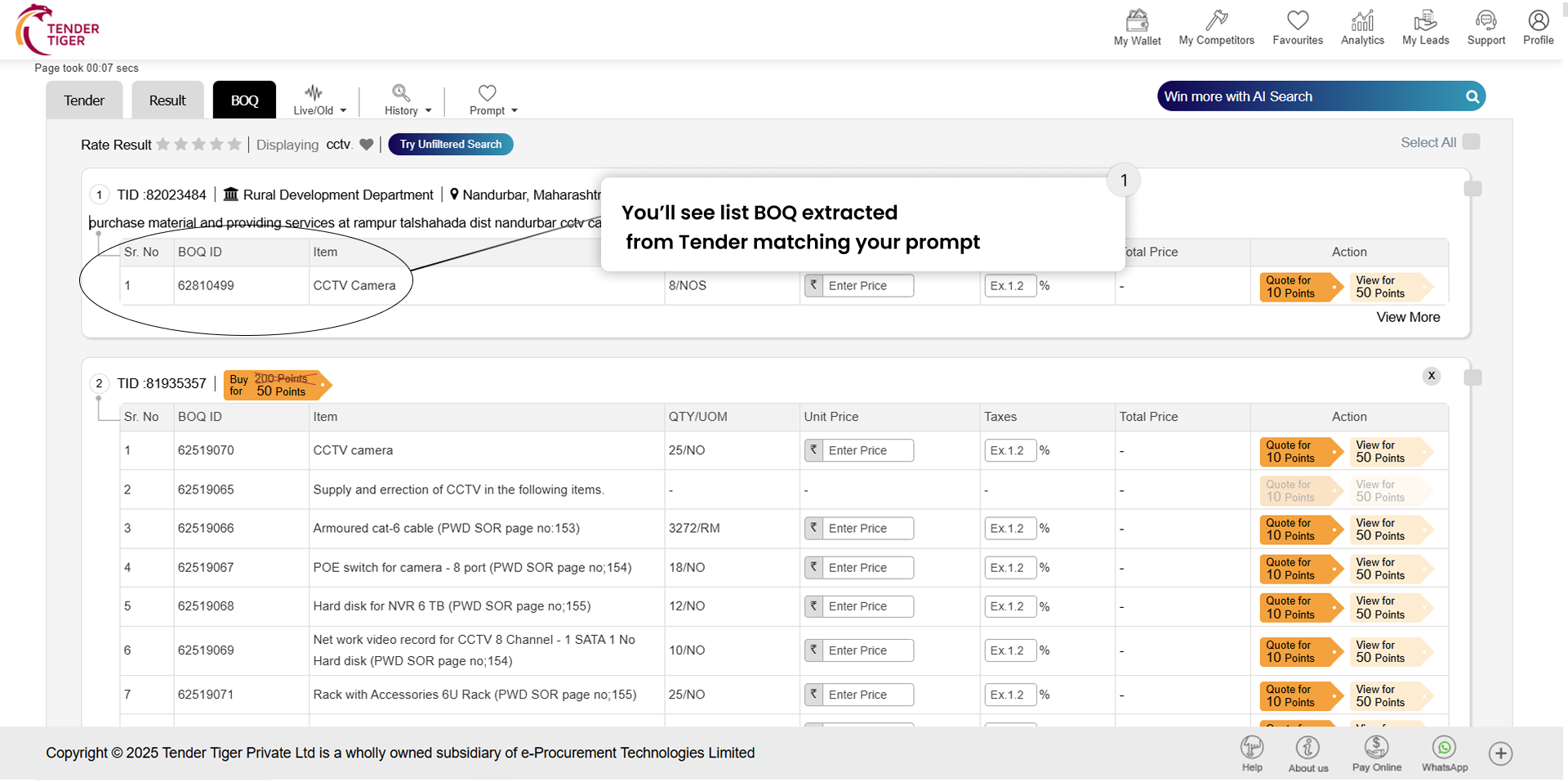
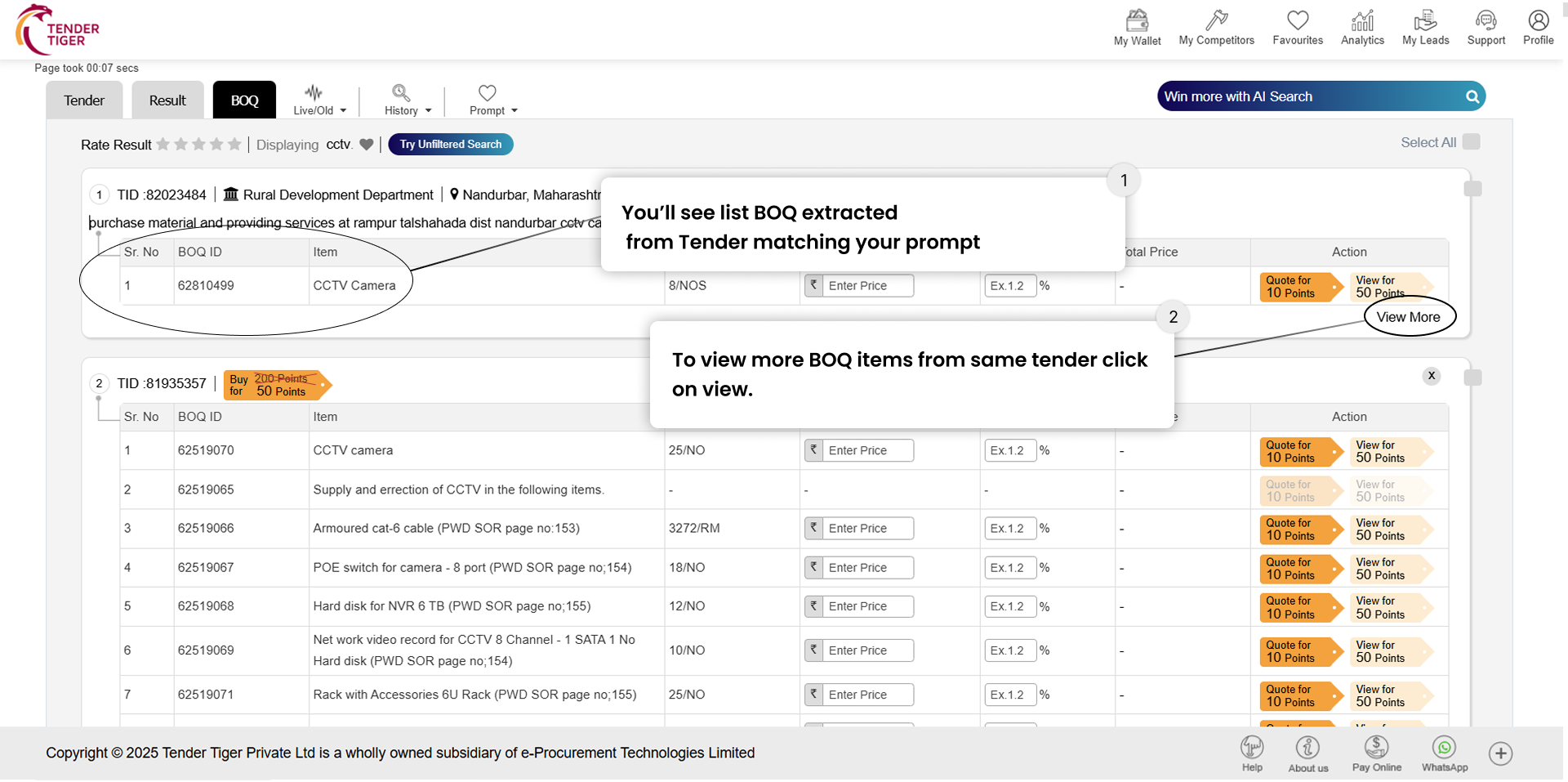
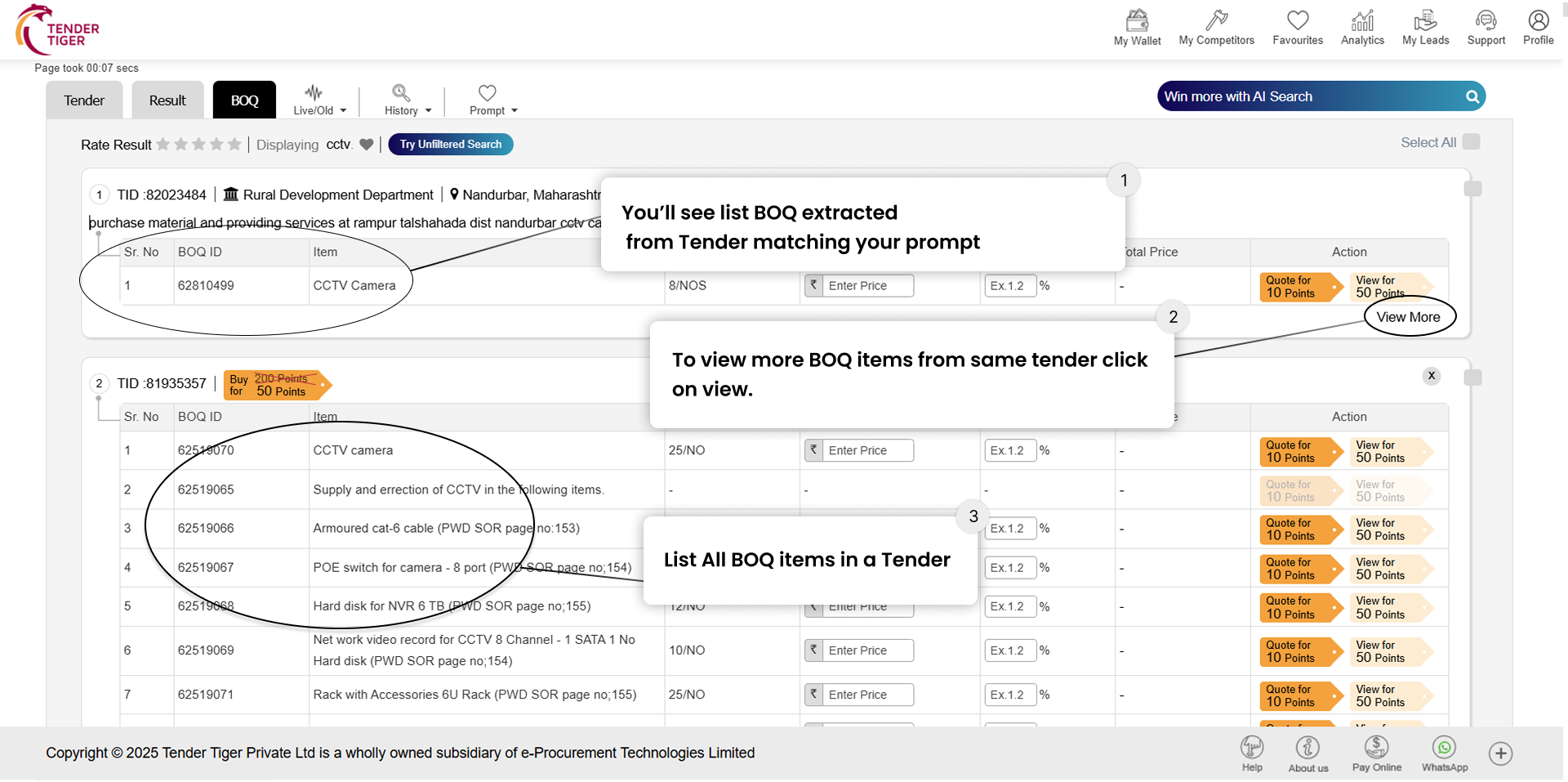
TRID :
8537292
Actual Tender Number :

TEC
TEC
TEC
Supply of drugs and dressings.
"metoclopramide tab/cap- , each tab/cap to contain:metoclopramide hcl 10mg", "antispasmodic drop/susp- , each ml to contain: dicyclomine hcl 10 mg, activated dimethicone 40mg, packing:10 ml bottle", "ciprofloxacin eye/ear drop- , each vial to contain: ciprofloxacin 0.3% w/v. , packing:5 to 10ml vial", "oxymetazoline nasal drop- , each vial to contain: oxymetazoline 0.05% w/v., packing:10ml vial", "xylometazoline nasal drop- , each vial to contain: xylometazoline 0.1% w/v., packing:10ml vial", "antacid tab/cap- , each tab/cap to contain :dried aluminium hydroxide gel 250 mg, magnesium hydroxide 250 mg, methyl polysiloxane/activated dimethicone 50 mg", "haemorrhoidal cream- , each tube to contain: beclomethasone dipropionate 0.025 %, phenylepherine hydrochloride 0.1 %, lignocaine hydrochloride 2.5 %., packing:20 gm tube", "analgesic gel- , each gm to contain: nimesulide 10mg, methyl salicylate 100mg, menthol 50mg, capaicin 0.25mg in cream base, packing:30gm tube", "ondansetron inj- , each ml to contain: ondansetron 2 mg, packing:4ml vial/amp", "saline nasal drop- , each phial to contain: sodium chloride 0.65%, benzalkonium chloride 0.001 %, phenyl mercuric acetate 0.002 %., packing:15ml vial", "sulphasalazine tab/cap- , each tab/cap to contain: sulphasalazine 500mg", "itopride tab/cap- , each tab/cap to contain: itopride hydrochloride 50mg", "pantoprazole inj- , each inj to contain: pantoprazole 40mg, packing: 1 vial/amp", "ursodesoxycholic acid tab/cap- , each tab/cap to contain: ursodesoxycholic acid 150mg.", "doxylamine + pyridoxin tab/cap- , each tab/cap to contain: doxylamine succinate 10mg, pyridoxin 10mg", "antacid gel- , each 5ml to contain: oxetacaine 10 mg, al. hydroxide 291 mg, mag. hydroxide 98 mg, packing:200ml bott", "laxative liquid- , each 15 ml to contain: milk of magnesia 11.25 ml, liquid paraffin 3.75 ml, packing:170ml bott", "drotaverine tab/cap- , each tab/cap to contain:drotavarine hcl 80 mg", "enzyme syp- , each 10 ml to contain: fungal diastase ( 1:2000) 40 mg, (aspergillus oryzae enzyme), cinnamon oil 0.25mg, caraway oil 0.5mg, cardamon oil 0.5 mg in glycerol sorbitol base., packing:200ml bott", "tramadol tab/cap- , each tab/cap to contain: tramadol hcl 50 mg", "ibuprofen oral susp- , each 5 ml to contain: ibuprofen 100 mg, packing:60ml bott", "ibuprofen and pcm oral susp- , each 5 ml to contain: ibuprofen 100 mg, paracetamol 162.5 mg, packing:60ml bott", "trypsin chymotrypsin tab/cap- , each tab/cap to contain: 1, 00, 000 armour units of enzymatic activity supplied by a purified concentrate which has specific trypsin and chymotrypsin activity in a ratio of approximately six to one", "antibiotic eye oint- , each gram to contain:polymyxin b sulphate 5000 iu, neomycin sulphate 3400 iu, zinc bacitracin 400 iu, hydrocortisone 10 mg in white sterilised petroleum jelly base., packing:5gm tube", "flurbiprofen eye drop- , eacl vial to contain: flurbiprofen 0.03% w/v, packing:5ml vial", "wax softner ear drop- , each vial to contain: para dichlorobenzene 2% w/v, benzocaine 2.7% w/v, chlorbutol 5%, turpentine oil 15% w/v, packing:10ml vial", "oxymetazoline nasal drop- , each vial to contain: oxymetazoline 0.025% w/v, packing:10ml vial", "budesonide nasal spray- , each actuation to deliver: budesonide 100 mcg., packing:150 doses", "laxative enema- , each bott to contain: glycerin i.p., 15%, sodium chloride i.p.15%, packing:30ml bott", "dicyclomine + mefenamic tab/cap- , each tab/cap to contain: dicyclomine 10mg, mefenamic acid 250mg", "thiocolchicoside tab/cap- , each tab/cap to contain: thiocolchicoside 4mg", "mebeverine tab/cap- , each tab/cap to contain: mebeverine hydrochloride 135mg", "hydroxy chloroquine tab/cap- , each tab/cap to contain: hydroxy chloroquine sulfate 200mg", "ornidazole tab/cap- , each tab/cap to contain: ornidazole 500mg", "drotaverine inj- , each ml to contain: drotaverine 20mg, packing:2ml vial/amp", "antacid gel- , each 5ml to contain: dried aluminium hydroxide gel 300mg magnesium hydroxide 250mg, activated simethicone 40mg, packing:170ml bott.", "liver disorders syp- , each 5ml to contain: tricholine citrate 250 mg, kalmegh ip eq. to andographolides 250mcg, sorbitrol-solution i.p. (70%) in a flavoured base, packing:200 ml bott", "liver disorders syp- , each 10ml to contain, tricholine citrate 0.55gm, sorbitol 7.15gm., packing:200 ml bott", "spray- , each pack to contain: spray diclofenac diethylmine 1.16%, methyl salicylate 10% w/w, linseed oil (oleum lini) 3% w/w, menthol 5% w/w, excipients & propellant q.s. 100 % w/w , packing:100gm ", "analgesic cream- , each tube to contain: cream methyl salicylate 8% w/w, mephenesin 5% w/w, methyl nicotinate 1% w/w, menthol 2% w/w, packing:20 gm tube", "gatifloxacin eye drop- , each vial to contain: gatifloxacin soln 0.3% w/v, packing:5ml vial", "moxifloxacin + prednisolone eye drop- , each ml to contain: moxifloxacin 0.5%, prednisolone acetate 1%, , packing:5ml vial", "travoprost eye drop- , each vial to contain: travoprost 0.004%, packing:2.5ml vial", "latanoprost eye drop- , each ml to contain: latanoprost 50mcg, aqueous buffered vehicle q.s, packing:2.5ml vial", "etanercept inj- , each pfp/pfs to contain:etanercept 50mg, packing: 1 pfs/pfp", "ranibizumab inj- , each ml to contain: ranibizumab 10 mg, packing:0.23 ml vial", "lactulose syp- , each 15ml to contain: lactulose 10gm, packing:200ml bott", "lactulose syp- , each 15ml to contain: lactulose 10gm, packing:450ml bott", "ondansetron syp - each 5ml to contain: ondansetron 2mg, packing:30ml bottle", "mefenamic acid tab/cap- , each tab/cap to contain : mefenamic acid 500mg", "haemorrhoidal cream- , each gm to contain: euphorbia prostrata dry extract ethanolic 80% (v/v) ((35-70):1) 10 mg (containing 0.315 -0.825 mg total flavonoids calcluated as apigenin-7 glucoside and 1.26-4.40 mg total phenolics calculated as gallic acid)cream base q.s., packing:30 gm tube", "tramadol and acetaminophen tab/cap- , each film coated tab/cap to contain: tramadol hydrochloride ip 18.75mg, acetaminophen ip 162.5mg", "tramadol and paracetamol tab/cap- , each tab/cap to contain: tramadol 37.50 mg, paracetamol 325mg", febuxostat tab - each tab to contain : febuxostat 40mg, "analgesic cream- , each tube to contain: methyl salicylate 30% w/w, menthol 10 % w/w, camphor 4 % w/w, excipients q.s., packing:30 gm tube", "besifloxacin eye drop-, each ml to contain: besifloxacin 0.6% (6mg/ml) , packing:5ml bott", "carboxy methyl cellulose eye drop- , each ml to contain: sodium carboxy methyl cellulose 5mg, packing:10ml vial", "travoprost and timolol eye drop- , each vial to contain: travoprost 0.004%, timolol 0.5%, packing:2.5ml vial", "hydroxypropylmethylcellulose eye drop- each vial to , contain: hydroxypropylmethylcellulose 0.3%, packing:5 to 10ml", "chlorhexidine soln- , each bott to contain: chlorhexidine gluconate 0.2% solution, packing:150ml bott", "potassium nit. & sod. monofluoro phosphate paste- , each tube to contain: potassium nitrate 5% w/w, sodium monofluoro phosphate 0.7% w/w, packing:100gm tube", "liver disorders syp- , each 15ml to contain: dl-methionine bp 100mg, choline dihydrogen citrate nfxii 100mg, l-leucine usp 19mg, l-isoleucine usp 10mg , l-valine usp11mg , pyridoxine hcl ip 1.5mg, nicotinamide ip 22.5mg, biotin usp 0.1mg , folic acid ip 0.5 mg, cyanocobalamin ip 3.0 mcg, inositol bp 50mg, packing:200ml bott", "liver disorders tab/cap-, each tab/cap to contain: dl-methionine bp 200mg , choline bitartrate (as coated) usp 200mg, l-leucine usp 19mg, l-isoleucine usp 10mg, l-valine usp 11mg, pyridoxine hydrochloride ip 1.5mg, nicotinamide ip 22.5mg, biotin usp 0.1mg, folic acid ip 0.5 mg, cyanocobalamin ip 3.0 mcg, inositol bp 50 mg", "lansoprazole tab/cap- , each orally disintegrated tab/cap to contain: lansoprazole 15mg (as enteric coated pellets)", "sucralfate susp- , each 5 ml to contain: sucralfate 1 g, packing:100ml bott.", "paracetamol tab/cap- , each tab/cap to contain: paracetamol 650mg" at
Classified in :